


















Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
Unlocking the Enigma: The Fascinating Kinetics of Chemical Processes

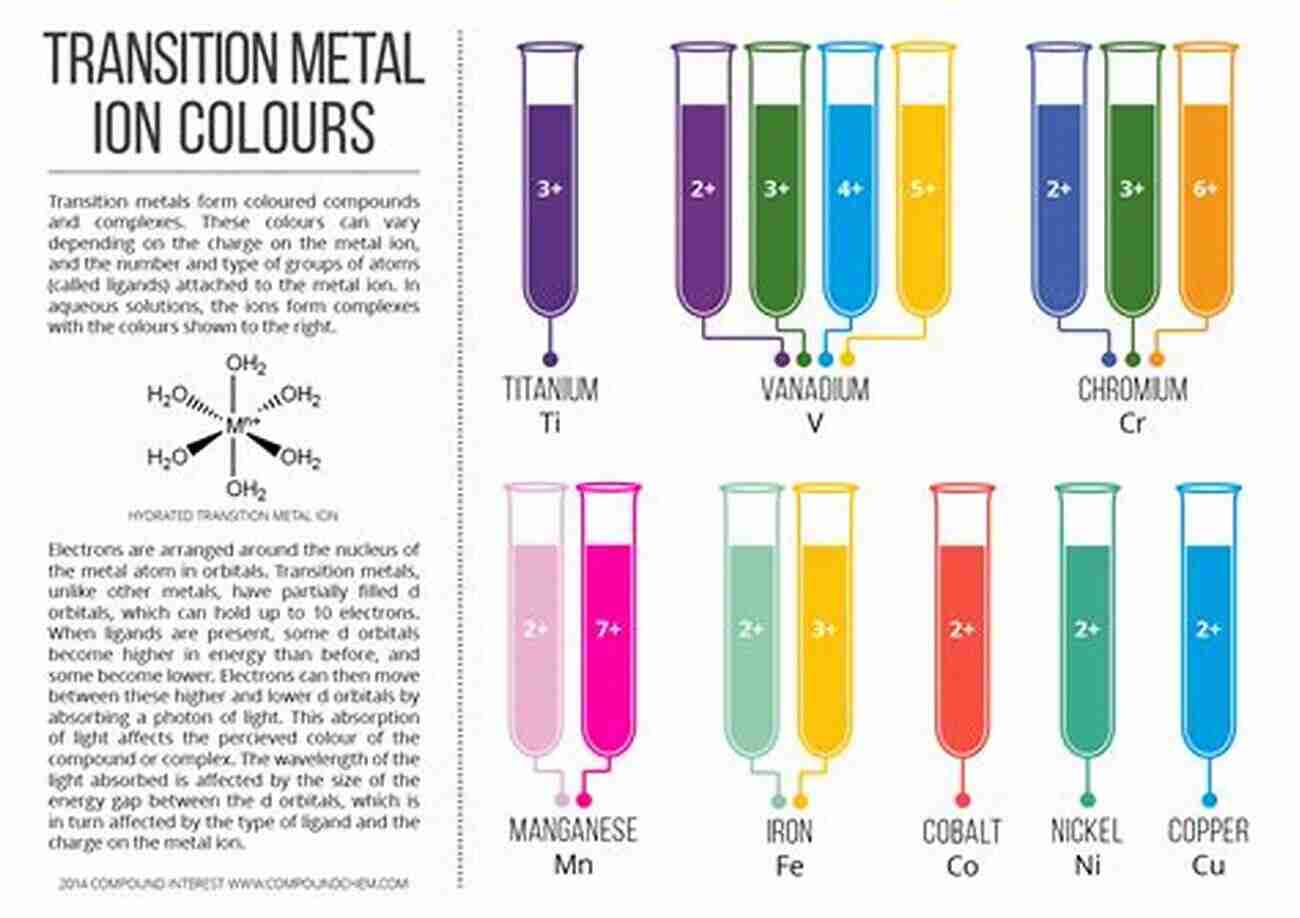
Chemistry, the science that studies the composition, properties, and transformations of matter, is a world filled with endless wonders. At the heart of chemical reactions lies a captivating facet known as kinetics. To unravel this intriguing concept, we delve into the depths of kinetics of chemical processes, exposing the secrets behind the speed of reactions and the factors that influence them.
What are Chemical Kinetics?
Chemical kinetics, a branch of physical chemistry, concerns itself with the rate at which chemical reactions occur and how they progress. It explores the factors that influence the speed of reactions, providing invaluable insights into the mechanisms that take place at the molecular level.
Alt attribute keyword: chemical reaction process producing colorful compounds
5 out of 5
| Language | : | English |
| File size | : | 20762 KB |
| Screen Reader | : | Supported |
| Print length | : | 262 pages |
The Basics: Reaction Rates and Rate Laws
The fundamental idea behind kinetics lies in understanding reaction rates and formulating rate laws. Reaction rates determine how quickly reactants transform into products, and rate laws establish mathematical relationships between the concentration of reactants and the rate at which the reaction occurs.
For example, in the reaction A + B → C, where A and B are reactants and C is the product, the rate of the reaction can be given by the equation: rate = k[A][B], where k is the rate constant and [A] and [B] represent the concentrations of A and B, respectively.
Factors Influencing Reaction Rates
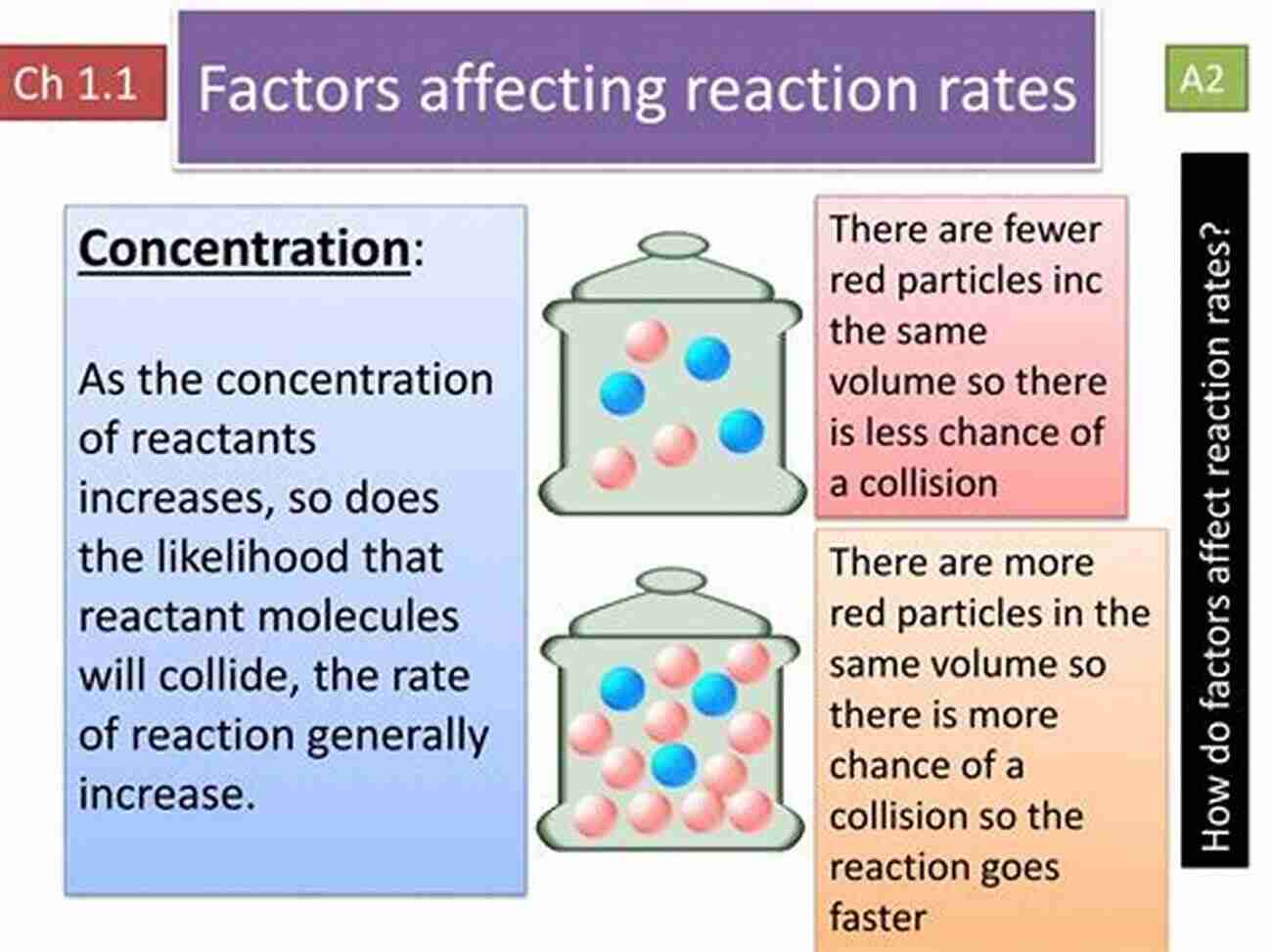
Understanding the factors that influence reaction rates is crucial in comprehending kinetics. These factors include temperature, concentration, surface area, catalysts, and the nature of reactants. By manipulating these variables, chemists can control the speed at which reactions occur and optimize processes in various industries.
Alt attribute keyword: Factors influencing reaction rates
1. Temperature
Temperature plays a significant role in reaction rates. As the temperature rises, the average kinetic energy of the molecules increases, resulting in more frequent and energetic collisions. This leads to a higher number of successful collisions, thereby enhancing reaction rates.
2. Concentration
The concentration of reactants is directly proportional to the likelihood of collisions. A higher concentration of reactants means that there are more particles available to collide with each other, increasing the chances of successful collisions and accelerating the reaction rate.
3. Surface Area
When solid reactants are involved, increasing their surface area exposes more particles to potential collisions. The larger the surface area, the more sites available for reactions to occur. Thus, finely divided or powdered reactants react faster than their bulk counterparts.
4. Catalysts
Catalysts are substances that speed up reactions by providing an alternative pathway with lower activation energy. They do not get consumed in the reaction but facilitate the conversion of reactants into products more rapidly. Industrial processes often rely on catalysts to improve efficiency and reduce costs.
5. Nature of Reactants
The chemical nature of the reactants influences the speed of reactions. Some substances have stronger bonds or more stable structures, making it more difficult for reactions to occur. Comparing different reactants allows scientists to study how molecular properties affect reaction rates.
Reaction Mechanisms and Rate-Determining Steps
Reaction mechanisms delve into the detailed steps involved in a chemical reaction. These mechanisms comprise elementary reactions, individual steps that combine to form the overall reaction. By identifying the rate-determining step, chemists can focus on understanding the slowest and most crucial step in the reaction.
Understanding reaction mechanisms provides opportunities for designing new catalysts, optimizing reaction conditions, and manipulating reaction pathways. It allows scientists to gain insight into complex systems and devise strategies for enhancing the efficiency of chemical processes.
The Role of Equilibrium in Kinetics
The concept of equilibrium, which pertains to the balance between reactants and products in a chemical system, also plays a crucial role in kinetics. By studying the equilibrium system and predicting directionality, chemists can deduce the extent to which a reaction has occurred at a given time.
Equilibrium constant expressions and rate constants share a complex relationship, offering a deeper understanding of the interplay between kinetics and equilibrium. It allows scientists to deduce the equilibrium position of a reaction based on the reaction rate and vice versa.
Applications of Kinetics in Everyday Life
Kinetics finds applications in various facets of everyday life, ranging from understanding the food we consume to developing life-saving medications and maintaining sustainable environments. Here are a few examples:
1. Food Preparation
Understanding the kinetics of cooking and baking allows chefs and home cooks to achieve the perfect taste and texture. By manipulating factors such as heat, time, and ingredient ratios, they can control the reactions occurring in the food, ensuring it turns out delicious.
2. Pharmaceuticals
Kinetics plays a crucial role in drug development, as it helps scientists understand how drugs interact with the body. By studying the speed at which a drug enters the bloodstream, distributes, and gets metabolized, they can optimize dosages and minimize side effects.
3. Environmental Conservation
Chemical reactions in environmental contexts, such as air pollution and water treatment, heavily rely on kinetics. By understanding the rates at which pollutants degrade or react with treatment chemicals, measures can be taken to safeguard the environment and ensure sustainable practices.
4. Industrial Processes
Virtually all industrial processes, from oil refining to manufacturing, utilize kinetics. By optimizing reaction rates, industries can enhance productivity, reduce costs, and reduce the environmental footprint of their operations. Understanding kinetics enables engineers to design efficient processes and improve overall performance.
Alt attribute keyword: Industrial processes utilizing kinetics
The kinetics of chemical processes is a bewitching domain that unravels the mysteries behind the speed of reactions. By exploring reaction rates, rate laws, and factors influencing reactions, scientists gain a deeper understanding of chemical processes and develop strategies to optimize them.
From cooking a delicious meal to developing life-saving medications, kinetics touches various facets of our lives, showcasing the immense impact that chemistry has on the world. By harnessing the knowledge of kinetics, we can unlock the potentials of chemical reactions and shape a brighter future.
5 out of 5
| Language | : | English |
| File size | : | 20762 KB |
| Screen Reader | : | Supported |
| Print length | : | 262 pages |
Kinetics of Chemical Processes details the concepts associated with the kinetic study of the chemical processes. The book is comprised of 10 chapters that present information relevant to applied research.
The text first covers the elementary chemical kinetics of elementary steps, and then proceeds to discussing catalysis. The next chapter tackles simplified kinetics of sequences at the steady state. Chapter 5 deals with coupled sequences in reaction networks, while Chapter 6 talks about autocatalysis and inhibition. The seventh chapter describes the irreducible transport phenomena in chemical kinetics. The next two chapters discuss the correlations in homogenous kinetics and heterogeneous catalysis, respectively. The last chapter covers the analysis of reaction networks.
The book will be of great use to students, researchers, and practitioners of scientific disciplines that deal with chemical reaction, particularly chemistry and chemical engineering.

 Fernando Pessoa
Fernando PessoaThe Ultimate Guide to New Addition Subtraction Games...
In this day and age, countless parents are...

 Ethan Mitchell
Ethan MitchellThe Ultimate Guide for the Aspiring Pianist: Unleash Your...
Are you a beginner pianist feeling...

 Gerald Parker
Gerald ParkerWow Robot Club Janice Gunstone - The Mastermind Behind...
Robots have always fascinated...

 Dylan Hayes
Dylan HayesIdeal For Catching Up At Home: CGP KS2 Geography
Are you looking for the perfect resource to...

 Kevin Turner
Kevin TurnerThe Ultimate Pictorial Travel Guide To Vietnam: Explore...
Discover the rich...

 D'Angelo Carter
D'Angelo CarterUnlocking the Secrets of Compact Stars: Exploring...
Compact stars have...

 Isaiah Price
Isaiah PriceUnveiling the Hidden Gem: Google Places Goliath Valley...
Are you tired of visiting the same old...
 Donald Ward
Donald WardEssays Towards Theory Of Knowledge: Exploring the Depths...
Are you ready to delve into...

 Thomas Mann
Thomas MannThe Ultimate PMP Project Management Professional All In...
Are you ready to take your project...

 Trevor Bell
Trevor Bell10 Incredible Stories From Life In Football That Will...
The Beautiful Game - Football...

 Zachary Cox
Zachary Cox100 Amazing And Unexpected Uses For Coconut Oil
Coconut oil, a versatile and widely loved...

 Owen Simmons
Owen SimmonsUnveiling the Enigma of Die Blaue Brosche: A Family’s...
Have you ever heard of Die Blaue Brosche...
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Raymond ParkerUnveiling the Secrets of Molecular Inorganic Chemistry: Exploring Structural...
Raymond ParkerUnveiling the Secrets of Molecular Inorganic Chemistry: Exploring Structural...
 Preston SimmonsColonial Conversions: Exploring Religious Transformations in French and...
Preston SimmonsColonial Conversions: Exploring Religious Transformations in French and...
 Roger TurnerThe Evolution of Space Mission Cameras and Instruments - A Look into Springer...
Roger TurnerThe Evolution of Space Mission Cameras and Instruments - A Look into Springer... Logan CoxFollow ·8.1k
Logan CoxFollow ·8.1k Israel BellFollow ·8.5k
Israel BellFollow ·8.5k Clay PowellFollow ·6.2k
Clay PowellFollow ·6.2k Abe MitchellFollow ·9.1k
Abe MitchellFollow ·9.1k Edison MitchellFollow ·18.7k
Edison MitchellFollow ·18.7k Carlos DrummondFollow ·17.8k
Carlos DrummondFollow ·17.8k Bradley DixonFollow ·10.8k
Bradley DixonFollow ·10.8k Patrick RothfussFollow ·17.8k
Patrick RothfussFollow ·17.8k














